
Insights+: EMA Marketing Authorization of New Drugs in October 2023
Shots:
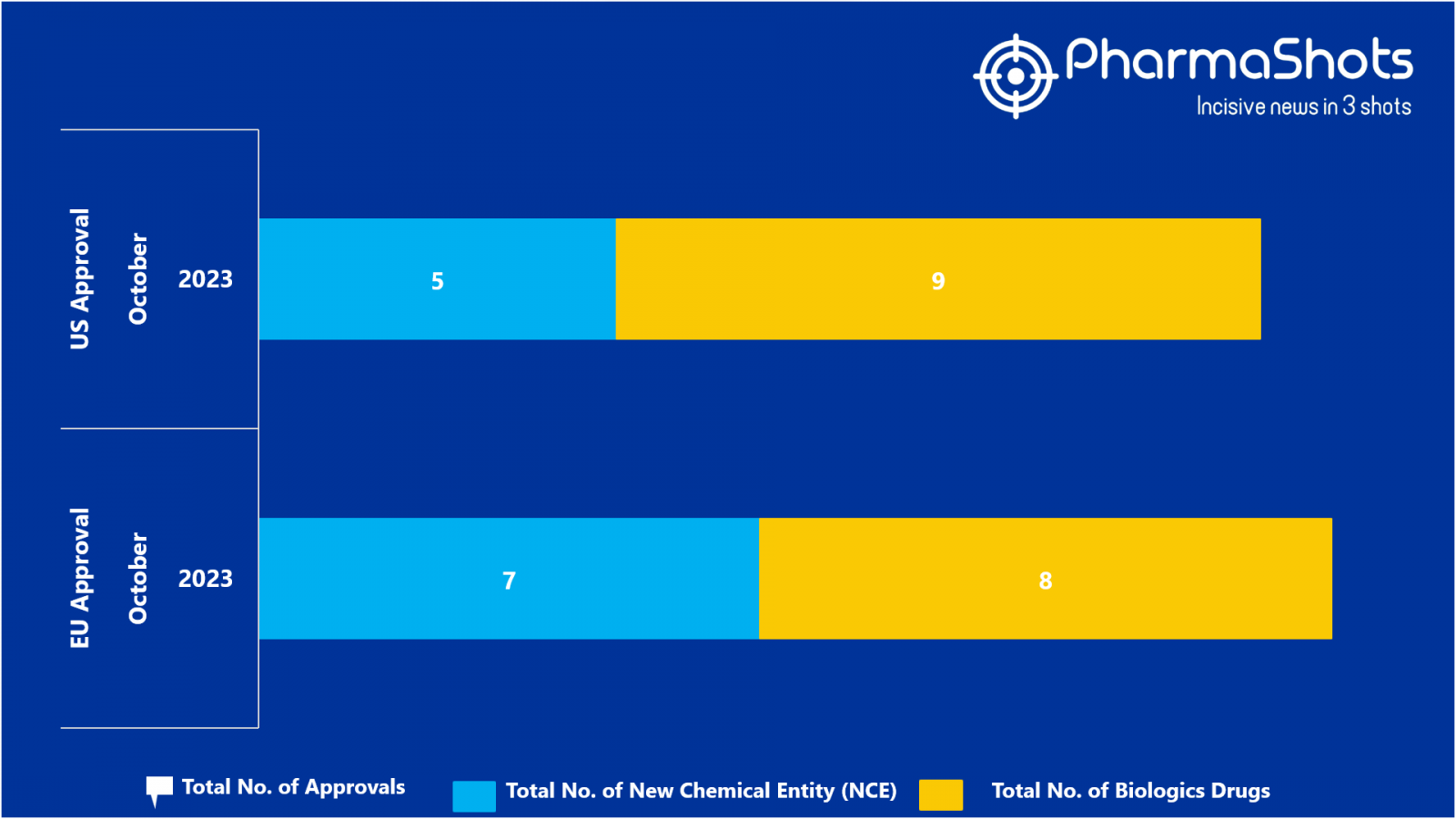
- The EMA approved 2 BLA while 7 new chemical Entity, 6 BLA received positive CHMP opinion in October 2023, leading to treatments for patients and advances in the healthcare industry
- In October 2023, the major highlight drugs were Keytruda + trastuzumab 1L treatment of LA unresectable or metastatic HER2+ gastric or GEJ adenocarcinoma & Adcetris + doxorubicin, vinblastine, and dacarbazine (AVD) to treat adult patients with previously untreated CD30+ Stage III Hodgkin lymphoma
- PharmaShots has compiled a list of a total of 15 new drugs approved by the EMA in October 2023
Sanofi's Praluent (Alirocumab) Received EU’s CHMP Positive Opinion
Praluent
Active ingredient: Alirocumab Date: Oct 12, 2023
Company: Sanofi Disease: Hypercholesterolemia
- The Positive Opinion was adopted on Alirocumab on patients (n=153) with Heterozygous Familial Hypercholesterolemia
- The P-III study primary objective evaluate the safety & efficacy of Alirocumab that were every Q2W & every Q4W vs PBO for 24 wks., whereas secondary objective includes effects on LDL-C levels, other lipid parameters & to evaluate safety & tolerability after open label treatment, additionally analysis of anti-alirocumab antibodies
- Praluent, which comes in 2 dosages (75 mg & 150 mg), is the first and only PCSK9 inhibitor authorized in the US which blocks PCSK9 from binding to LDLR & decreases blood levels of LDL-Cl and increases the number of LDLRs available to remove LDL
Guerbet and Bracco Received Positive Opinion From EU’s CHMP for Elucirem & Vueway (Gadopiclenol)
Elucirem & Vueway
Active ingredient: Gadopiclenol Date: Oct 12, 2023
Company: Guerbet and Bracco Disease: N/A
- The positive opinion was adopted based on 2 P-III Clinical trials (PICTURE) & (PROMISE) evaluating efficacy & safety for Elucirem & Vueway, MRI with contrast enhancement in adults & children aged 2yrs. & over
- The result of the study demonstrated that Gadopiclenol provided non-inferior outcomes in brain & whole body. The evaluation criteria were met with Gadopiclenol (0.05 mmol/kg) injection which includes the superiority of using a contrast product in an examination over one without & the non-inferiority of Gadopiclenol (0.05 mmol/kg) vs Gadobutrol (0.1 mmol/kg)
- Gadopiclenol is a novel macrocyclic gadolinium-based contrast agent (GBCA) with excellent relaxivity. Its efficacy and safety have been assessed in MRI scans of the musculoskeletal system, head, neck, thorax, abdomen, and pelvis
Imfinzi
Active ingredient: Durvalumab Date: Oct 12, 2023
Company: AstraZeneca Disease: Hepatocellular Carcinoma
- The positive opinion was adopted for the new indication of Imfinzi (durvalumab) as 1L monotx. for the treatment of advanced or unresectable hepatocellular carcinoma was based on a P-III study (HIMALAYA)
- The P-III (HIMALAYA) clinical trial evaluates the safety & efficacy of Imjudo (300mg) + Imfinzi (1500, q4w) vs sorafenib in unresectable HCC patients (n=1324) with no prior systemic therapy
- Imfinzi (durvalumab) is a human PD-L1 protein and blocks PD-1 and CD80 proteins. Imfinzi in combination with Imjudo (tremelimumab) approved in unresectable HCC in the US, EU, Japan and many other countries based on the TOPAZ-1 and HIMALAYA study
Veyvondi
Active ingredient: Vonicog alfa Date: Oct 12, 2023
Company: Baxalta Disease: Bleeding episodes in von Willebrand
- The positive opinion was for the extension of indication to include "prophylactic treatment to prevent or reduce the frequency of bleeding episodes based on the results from study 071301 & study SHP677-304
- Study 071301 is a prospective, P-III, global, multicentred study on efficacy and safety of prophylaxis with rVWF in severe von Willebrand disease whereas study SHP677-304 is a P-IIIB, prospective study to assess the long-term safety and efficacy of rVWF in paediatric and adult subjects with severe von Willebrand disease
- Veyvondi is an is a recombinant von Willebrand factor. It has been approved in EU since Sep 2018 in adult patients with von Willebrand Disease (VWD), when desmopressin (DDAVP) treatment alone is ineffective or not indicated for the treatment of haemorrhage and surgical bleeding & prevention of surgical bleeding
Agamree
Active ingredient: vamorolone Date: Oct 13, 2023
Company: Santhera Disease: Duchenne Muscular Dystrophy
- The EMA’s CHMP has adopted a positive opinion recommending approval of Agamree for DMD patients aged ≥4yrs. The EC’s decision is expected within ~2mos. The MAA will be valid in all 27 member states of the EU as well as Iceland, Liechtenstein & Norway
- The clinical evidence was based on the (VISION-DMD) study & 3 open-label studies of vamorolone (given at doses b/w 2 & 6mg/kg/day for ~30mos.) as well as (FOR-DMD) study & several clinical pharmacology studies
- In the (VISION-DMD) study, boys treated with vamorolone on average growth maintained similar to PBO & those with prednisone on avg. experienced growth stunting, patients were able to resume growing in height who switched from prednisone to vamorolone after 24wks. The product is expected to be available in Germany in Q1’24
Pfizer Received Positive EU’S CHMP Opinion for Elrexfio to Treat Multiple Myeloma
Elrexfio
Active ingredient: Elranatamab Date: Oct 13, 2023
Company: Pfizer Disease: Multiple Myeloma
- The CHMP's positive opinion was based on Cohort A of P-II (MagnetisMM-3) evaluating Elrexfio for r/r Multiple Myeloma patients
- The P-II study results depicted OR of 61% & 72% chance of maintaining response at 15mos., furthermore, after switching to Q2W dosage at least 6mos. before the data cut-off date (n = 50), 38% of responding patients had a complete response (CR), and 80% of patients maintained or improved their response
- Elrexfio (SC formulation) that combines B-cell maturation antigen (BCMA)-CD3-directed BsAbs to kill myeloma cells by binding to BCMA on the cells and CD3 on the T-cells
Immedica’s Loargys Received positive opinion from EU’s CHMP to treat arginase-1 deficiency (ARGI-D)
Loargys
Active ingredient: Pegzilarginase Date: Oct 13, 2023
Company: Immedica Disease: Arginase-1 deficiency
- The positive opinion was based on a P-III study (PEACE) evaluating Pegzilarginase vs PBO to treat ARGI-D in patients 2yrs older & above
- The P-III (PEACE) trial met its 1EPs i.e., 76.7% reduction in mean plasma arginine over PBO, 90.5% achieved normal plasma arginine levels, and no discontinuations due to TRAEs while the P-I/II & P-II OLE study results showed clinical improvements, sustained lowering of plasma arginine & improvements in measures of mobility
- Loargys (Pegzilarginase) a new recombinant human enzyme, showed enhanced clinical outcomes by quickly and persistently lowering plasma levels of the amino acid arginine and its hazardous byproducts
Rezzayo
Active ingredient: Rezafungin Date: Oct 13, 2023
Company: Cidara Therapeutics & Mundipharma Disease: Invasive Candidiasis
- The positive opinion was based on a P-III (ReSTORE) evaluating the safety & efficacy of Rezafungin vs SoC (caspofungin) on patients with invasive candidiasis in 1:1 ratio
- The results of 1EP of the study showed a day 14 global cure rate of 59.1% vs 60.6% & the result of 2EP includes day 5 Mycologic eradication of 78.1% vs 68.7%; day 5 global cure of 55.9% vs 52.1%; day 14 mycologic eradication of 71.9% vs 70.1%
- Additionally exploratory endpoint showed day 1 -ve blood culture in 53.7% vs 46.2%; day 2 -ve blood culture in 74.2% vs 64.1% along with median ICU stay length of 5 vs 14.5 days
BeiGene’s Brukinsa Receive Positive Opinion from EU’s CHMP for the treatment of Follicular Lymphoma
Brukinsa
Active ingredient: Zanubrutinib Date: Oct 13, 2023
Company: BeiGene Disease: Follicular Lymphoma
- The Positive opinion was based on P-II study (ROSEWOOD) evaluating Brukinsa+ obinutuzumab vs obinutuzumab alone in r/r Follicular Lymphoma patients (n=217)
- The results from the study depicted an ORR of 69% vs 45.8% with a median follow-up of ~20mos. whereas DoR was found to be 69.3% in patients being treated with the combination therapy & mPFS was 28.0mos. vs 10.4mos.
- Brukinsa, a BTK inhibitor, has been approved as a monotx. in the EU & the company has planned submissions for the regulatory review of Brukinsa by the US FDA & NMPA. Moreover, the applications for Brukinsa are under review by the regulatory authorities of Canada, Switzerland & the UK
Veoza
Active ingredient: fezolinetant Date: Oct 13, 2023
Company: Astella Disease: VMS associated with Menopause
- The EMA’s CHMP has adopted a positive opinion recommending the use of Veoza (fezolinetant) 45 mg QD for the treatment of moderate to severe vasomotor symptoms (VMS) also known as hot flashes and/or night flashes associated with menopause. The EC’s decision is expected by YE 2023
- The positive opinion was adopted based on 3 P-III studies from BRIGHT SKY Program which included (SKYLIGHT 1), (SKYLIGHT 2) & (SKYLIGHT 4) evaluating the safety & efficacy of Veoza (fezolinetant) to treat patients (n=3000) suffering from moderate to severe VMS-associated with menopause
- Fezolinetant is a selective neurokinin-3 (NK3) receptor antagonist, which blocks NKB by binding on the kisspeptin/neurokinin B/dynorphin (KNDy) neuron, helping restore the balance in the brain’s temperature control center (the hypothalamus) to reduce the number and intensity of hot flashes and night sweats
Jemperli
Active ingredient: Dostarlimab Date: Oct 16, 2023
Company: GSK Disease: Endometrial Cancer
- The EMA’s CHMP has adopted a positive opinion recommending approval of Jemperli + carboplatin-paclitaxel for adult patients with dMMR/MSI-H primary advanced or recurrent EC. The EC’s decision is expected at the end of 2023
- The opinion was based on interim analysis results from part 1 of the P-III trial (RUBY/ENGOT-EN6/GOG3031/NSGO) evaluating Jemperli + carboplatin-paclitaxel vs carboplatin-paclitaxel + PBO. Part 1 of the trial met its 1EPs of investigator assessed PFS & showed a 72% reduction in risk of disease progression or death
- The results were presented at ESMO and SGO 2023 and published in the NEJM. If Jemperli is approved, it will become 1st new frontline treatment option in the EU & the immuno-oncology combination regimen available for a patient population
Keytruda
Active ingredient: Pembrolizumab Date: Oct 17, 2023
Company: Merck Disease: Gastric Cancer
- The EC has approved Merck’s Keytruda in combination with trastuzumab, fluoropyrimidine- and Pt-containing CT as 1L treatment of LA unresectable or metastatic HER2+ gastric or GEJ adenocarcinoma in adults whose tumors express PD-L1
- The approval was based on results from the P-III (KEYNOTE-811) evaluating Keytruda (200mg, q3w) + trastuzumab and CT vs trastuzumab and CT alone in 732 patients which showed significant improvement in PFS, & ORR, ≥80% of patients with PD-L1+ tumors
- Trend toward improvement in OS in the ITT population but these results did not meet statistical significance per the pre-specified statistical analysis plan. The results will be presented at ESMO 2023
Merck’s Prevymis Received positive EU’s CHMP Opinion for the prophylaxis of cytomegalovirus
Prevymis
Active ingredient: Letermovir Date: Oct 17, 2023
Company: Merck Disease: Cytomegalovirus
- The EMA's CHMP adopted positive opinion recommending Prevymis (letermovir) for CMV prophylaxis in high-risk adult kidney transplant recipients & extends its dosing from 100 to 200 days for bone marrow transplant pts who are at risk for late CMV infection. EC’s final decision is expected by YE 2023
- The opinion regarding use of prevymis as CMV disease prophylaxis was based on P-III trial (NCT03443869) having 567 high risk adult kidney transplant recipients. Additionally, the opinion of extending Prevymis dosing from 14 wks. to 28 wks. post HSCT was based on another P-III trial (NCT03930615)
- Prevymis is an antiviral agent already approved by FDA for CMV disease prophylaxis in high-risk adult kidney transplant pts. and has extended 200-day dosing as CMV prophylaxis in HSCT recipients
Rubraca Received EU’s CHMP Positive Opinion for the treatment of Ovarian Cancer
Rubraca
Active ingredient: Rucaparib camsylate Date: Oct 17, 2023
Company: Pharmaand Disease: Ovarian Cancer
- The EMA’s CHMP has adopted a positive opinion recommending approval of a Type II variation for Rubraca (rucaparib), as 1st L maintenance treatment for all women with advanced ovarian cancer regardless of their BRCA mutation status, following frontline platinum-based CT. The EC’s decision is expected by YE 2023
- The opinion was based on the P-III ATHENA-MONO trial (NCT03522246) evaluating rucaparib monotx, vs PBO in 538 women with newly diagnosed ovarian cancer showing improved investigator-assessed PFS and similar safety profiles for U.S. and European labels of rucaparib
- Rubraca is a PARP-1 inhibitor targeting the DNA repair mechanism in genetically mutated cancer cells, leading to cancer cell death & reduced tumor growth
Adcetris
Active ingredient: Brentuximab vedotion Date: Oct 19, 2023
Company: Takeda Disease: Hodgkin Lymphoma
- The EC has approved Adcetris in combination with doxorubicin, vinblastine, and dacarbazine (AVD) to treat adult patients with previously untreated CD30+ Stage III Hodgkin lymphoma
- The approval was based on the P-III trial (ECHELON-1) evaluating Adcetris (ADC directed at CD30) + AVD vs doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) in adult patients. The trial met its 1EPs of modified PFS, as well as 2EPs of OS & showed a significant improvement in OS in adult patients with previously untreated Stage III or IV classical Hodgkin lymphoma
- The safety profile was consistent with prior studies with no new safety signals. Adcetris received marketing authorization from regulatory authorities in 70+ countries for r/r Hodgkin lymphoma and sALCL
Related Post:
Tags

Kritika is a content writer at PharmaShots. She is interested in covering recent innovations from the pharma & MedTech industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.














